[ad_1]
The European Commission, on behalf of member states, has sealed deals with six companies for up to 2.3 billion vaccine doses. The BioNTech-Pfizer and Moderna vaccines are so far the only jabs authorised in the EU, but the vaccine jointly developed by Oxford and AstraZeneca is expected to be approved on Friday (29 January) by the European Medical Agency (EMA).
With two doses per person needed for both authorised vaccines, the EU could vaccinate at least 380 million people – covering approximately 80 percent of the European population.
-

The EU has sealed deals with six companies for up to 2.3 billion vaccine doses (Photo: European Council)
The EU Commission from August 2020 until January 2021 signed so-called “advanced purchase agreements” with six different companies, and secured 2.3bn doses. It started work on the agreements at a time when it was still unclear which company or companies would be the first to come up with an effective and safe vaccine.
The commission described these agreements as investments, with the money transferred to the companies in tranches depending on delivery. The aim was to spread the risk, “diversify the portfolio”: betting on several vaccines at the same time to speed up vaccine development and/or manufacturing. The idea also was to avoid individual EU countries competing with each other. The vaccines belong to the member states, the EU does not have doses.
Why are the contracts not public?
The commission is holding vaccine contracts in secrecy, relying on confidentiality clauses and the need to protect commercial interests. Five-out-of-six contracts remain confidential. After months of growing pressure, the commission invited MEPs to read a redacted version of the CureVac contract under strict conditions – although a few days later it then decided to post the redacted CureVac contract on its website. The European Ombudsman has launched an investigation into the commission’s refusal to give the public access to contracts.
Are there separate deals carried out by member states?
Germany has secured 30 million additional vaccine doses for its own citizens under a separate agreement with BioTech – an announcement that explicitly appears on the German health ministry website. While member states agreed “not to launch their own procedures for advance purchase of that vaccine with the same manufacturers,” the commission has refused so far to clarify whether the German deal is legal under the EU vaccine strategy.
Meanwhile, Cyprus said that it is in talks with Israel about a bilateral arrangement as well, while Hungary has secured two million doses of Russian Sputnik vaccine.
Who is paying for these vaccines in the EU?
The EU has invested €2.7bn in the rapid development and research of the vaccines with various companies, but member states themselves buy the vaccine doses from the companies directly. Once a vaccine is considered safe and effective by the EMA, member states can place their orders and pay directly, depending on how many doses were allocated to them based on their population size – without the commission’s involvement.
How much do the vaccines cost?
It was not officially disclosed how much member states pay for the vaccine. But the Belgian budget state secretary Eva De Bleeker accidentally tweeted the price for each vaccine the EU has signed agreements for.
Of the two vaccines, which had been approved by EMA: Pfizer/BioNTech cost €12, and Moderna €15. AstraZeneca has pledged to sell the vaccine at a price that covers their costs, resulting in a €1.78 price tag. The Johnson & Johnson jab costs €7, and the CureVac €10. The commission did not confirm the figures but said since companies received money for certain expenditure regarding vaccine production, member states would eventually have to pay less.
Who is responsible for the deliveries?
Logistics and transportation also depend on member states. Once the doses are produced, it is up to the EU countries and the specific companies to agree on the exact deliveries. Some members states have opted for one hub and others have opened distribution to several hubs.
When will vaccines be widely available in Europe?
Under EU contracts, the majority of vaccines delivery is foreseen to be completed in 2021. The largest quantities of vaccines are expected during the second quarter of 2021 (April to June), as already agreed in the existing contracts. However, the recent delays that both Pfizer-BioNtech and AstraZeneca have announced to the deliveries to the EU have raised questions about possible shortfalls occurring also during the second quarter of this year.
How many people need to get vaccinated to have herd immunity? What about mutations?
For known diseases, a herd immunity allowing control of a pandemic requires around 70 percent of the population to have protection. The commission wants member states to vaccinate at least 70 percent of the adult population by the end of summer. The World Health Organization, however, predicted that, globally, herd immunity will not be achieved in 2021.
It is as yet unclear how long vaccines prevent serious illness. Companies are adapting the vaccines to respond to new mutations. Moderna said in January that its vaccine protects against the ‘South African’ variant, but acknowledged that antibodies triggered by the vaccine seem less potent against the new variant.
How is EMA making Covid-19 authorisations? Is it slow?
The EMA is carrying out a fast-track but standardised regulatory process for the approval of vaccines candidates that have applied for EU-wide authorisation. All data from clinical trials is examined as it becomes available to accelerate the process. After monitoring the results, EMA has to issue a scientific opinion that the commission uses to discuss with member states and give the official green light to the authorisation. Under this approach, the same standards of quality, safety and efficacy for the approval of all EU medicines will apply to Covid-19 vaccines.
Meanwhile, the UK has achieved some notoriety for approving vaccines quicker than the EU. But the procedure followed by the British authorities, for example for the approval of Pfizer-BioNTech jab, gives the companies complete immunity from legal liability in civil cases. Under EU law, all member states can opt for this license, known as an “emergency-use authorisation”.
With its order for the Russian vaccine, Hungary is the first EU member to approve unilaterally a vaccine that is not authorised by the EMA.
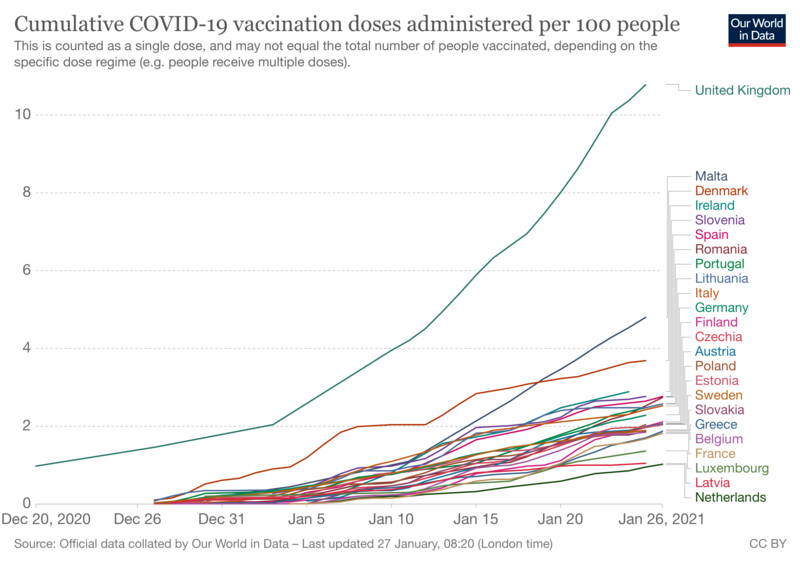
How different are member states’ vaccination programmes and why?
EU countries design their own vaccination logistics, programs and set up their own priorities, which results in different paces of roll-outs in member states. The delivery to a national distribution hub or hubs is done by the manufacturers.
The commission last October suggested EU countries prepare: with skilled workforce, medical and protective equipment, transportation and storage capacities, and clear communication. The commission also suggested that member states prioritise healthcare and care workers, persons over 60 years and with health risks, essential workers. These are not mandatory guidelines, but EU countries, in general, started inoculations with their care workers and elderly.
Will vaccinations be required at work or to travel?
Greek prime minister Kyriakos Mitsotakis first suggested creating vaccine certificates to allow travel across the bloc and save the 2021 summer for tourism. The EU will roll out a common approach so that a certificate issued in one member states is recognised in another. But it is not decided yet if they will be used for travelling, less alone be mandatory. Especially since people could still infect others despite being vaccinated. Workplaces might decide to give incentives for employees to get vaccinated, but it is unlikely that it would be mandatory.
[ad_2]
Source link